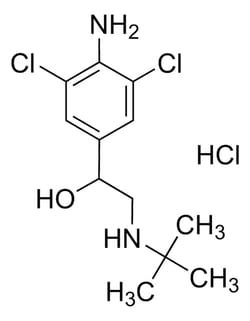
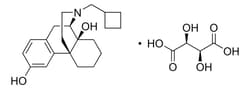
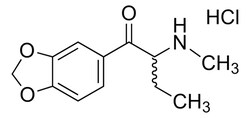
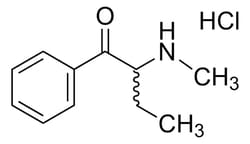
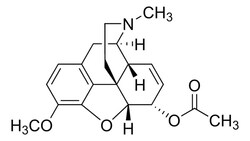
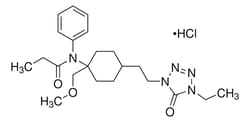
Drug Standards
- (1)
- (3)
- (13)
- (563)
- (3)
- (1)
- (24)
- (35)
- (1)
- (1)
- (1)
- (4)
- (9)
- (1)
- (1)
- (12)
- (2)
- (14)
- (1)
- (1)
- (3)
- (1)
- (1)
- (18)
- (3)
- (18)
- (1)
- (2)
- (1)
- (29)
- (2)
- (6)
- (1)
- (1)
- (1)
- (1)
- (1)
- (1)
- (2)
- (2)
- (1)
- (2)
- (1)
- (2)
- (1)
- (2)
- (1)
- (1)
- (2)
- (1)
- (3)
- (1)
- (2)
- (1)
- (1)
- (2)
- (1)
- (1)
- (2)
- (1)
- (2)
- (1)
- (2)
- (2)
- (1)
- (2)
- (2)
- (2)
- (1)
- (2)
- (2)
- (1)
- (2)
- (2)
- (2)
- (2)
- (1)
- (1)
- (2)
- (2)
- (1)
- (1)
- (1)
- (1)
- (1)
- (2)
- (2)
- (2)
- (1)
- (1)
- (1)
- (2)
- (1)
- (1)
- (1)
- (2)
- (1)
- (1)
- (1)
- (1)
- (2)
- (20)
- (4)
- (1)
- (1)
- (1)
- (2)
- (10)
- (7)
- (1)
- (7)
Filtered Search Results
Montelukast sodium, 98%
CAS: 151767-02-1 Molecular Formula: C35H35ClNNaO3S Molecular Weight (g/mol): 608.17 MDL Number: MFCD00931431 InChI Key: LBFBRXGCXUHRJY-HKHDRNBDSA-M PubChem CID: 23663996 ChEBI: CHEBI:6993 IUPAC Name: sodium;2-[1-[[(1R)-1-[3-[(E)-2-(7-chloroquinolin-2-yl)ethenyl]phenyl]-3-[2-(2-hydroxypropan-2-yl)phenyl]propyl]sulfanylmethyl]cyclopropyl]acetate SMILES: [Na+].CC(C)(O)C1=CC=CC=C1CC[C@@H](SCC1(CC([O-])=O)CC1)C1=CC=CC(\C=C\C2=CC=C3C=CC(Cl)=CC3=N2)=C1
| PubChem CID | 23663996 |
|---|---|
| CAS | 151767-02-1 |
| Molecular Weight (g/mol) | 608.17 |
| ChEBI | CHEBI:6993 |
| MDL Number | MFCD00931431 |
| SMILES | [Na+].CC(C)(O)C1=CC=CC=C1CC[C@@H](SCC1(CC([O-])=O)CC1)C1=CC=CC(\C=C\C2=CC=C3C=CC(Cl)=CC3=N2)=C1 |
| IUPAC Name | sodium;2-[1-[[(1R)-1-[3-[(E)-2-(7-chloroquinolin-2-yl)ethenyl]phenyl]-3-[2-(2-hydroxypropan-2-yl)phenyl]propyl]sulfanylmethyl]cyclopropyl]acetate |
| InChI Key | LBFBRXGCXUHRJY-HKHDRNBDSA-M |
| Molecular Formula | C35H35ClNNaO3S |
9-beta-D-Arabinofuranosylguanine, Thermo Scientific™
CAS: 38819-10-2 Molecular Formula: C10H13N5O5 Molecular Weight (g/mol): 283.24 MDL Number: MFCD00065486 InChI Key: NYHBQMYGNKIUIF-FJFJXFQQSA-N Synonym: Araguanosine; Ara-G IUPAC Name: 2-amino-9-[(2R,3S,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-6,9-dihydro-3H-purin-6-one SMILES: NC1=NC(=O)C2=C(N1)N(C=N2)[C@@H]1O[C@H](CO)[C@@H](O)[C@@H]1O
| CAS | 38819-10-2 |
|---|---|
| Molecular Weight (g/mol) | 283.24 |
| MDL Number | MFCD00065486 |
| SMILES | NC1=NC(=O)C2=C(N1)N(C=N2)[C@@H]1O[C@H](CO)[C@@H](O)[C@@H]1O |
| Synonym | Araguanosine; Ara-G |
| IUPAC Name | 2-amino-9-[(2R,3S,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-6,9-dihydro-3H-purin-6-one |
| InChI Key | NYHBQMYGNKIUIF-FJFJXFQQSA-N |
| Molecular Formula | C10H13N5O5 |
Etravirine, Thermo Scientific™
CAS: 269055-15-4 Molecular Formula: C20H15BrN6O Molecular Weight (g/mol): 435.29 MDL Number: MFCD09837879 InChI Key: PYGWGZALEOIKDF-UHFFFAOYSA-N Synonym: 4-((6-Amino-5-bromo-2-((4-cyanophenyl)amino)pyrimidin-4-yl)oxy)-3,5-dimethylbenzonitrile IUPAC Name: 4-({6-amino-5-bromo-2-[(4-cyanophenyl)amino]pyrimidin-4-yl}oxy)-3,5-dimethylbenzonitrile SMILES: CC1=CC(=CC(C)=C1OC1=NC(NC2=CC=C(C=C2)C#N)=NC(N)=C1Br)C#N
| CAS | 269055-15-4 |
|---|---|
| Molecular Weight (g/mol) | 435.29 |
| MDL Number | MFCD09837879 |
| SMILES | CC1=CC(=CC(C)=C1OC1=NC(NC2=CC=C(C=C2)C#N)=NC(N)=C1Br)C#N |
| Synonym | 4-((6-Amino-5-bromo-2-((4-cyanophenyl)amino)pyrimidin-4-yl)oxy)-3,5-dimethylbenzonitrile |
| IUPAC Name | 4-({6-amino-5-bromo-2-[(4-cyanophenyl)amino]pyrimidin-4-yl}oxy)-3,5-dimethylbenzonitrile |
| InChI Key | PYGWGZALEOIKDF-UHFFFAOYSA-N |
| Molecular Formula | C20H15BrN6O |
Febantel, Thermo Scientific™
CAS: 58306-30-2 Molecular Formula: C20H22N4O6S Molecular Weight (g/mol): 446.48 MDL Number: 01738527 InChI Key: HMCCXLBXIJMERM-UHFFFAOYSA-N IUPAC Name: methyl N-{[(methoxycarbonyl)imino]({[2-(2-methoxyacetamido)-4-(phenylsulfanyl)phenyl]amino})methyl}carbamate SMILES: COCC(=O)NC1=CC(SC2=CC=CC=C2)=CC=C1NC(NC(=O)OC)=NC(=O)OC
| CAS | 58306-30-2 |
|---|---|
| Molecular Weight (g/mol) | 446.48 |
| MDL Number | 01738527 |
| SMILES | COCC(=O)NC1=CC(SC2=CC=CC=C2)=CC=C1NC(NC(=O)OC)=NC(=O)OC |
| IUPAC Name | methyl N-{[(methoxycarbonyl)imino]({[2-(2-methoxyacetamido)-4-(phenylsulfanyl)phenyl]amino})methyl}carbamate |
| InChI Key | HMCCXLBXIJMERM-UHFFFAOYSA-N |
| Molecular Formula | C20H22N4O6S |