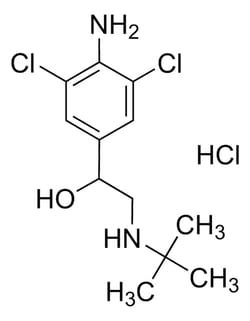
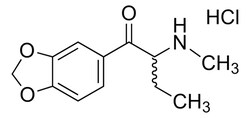
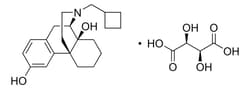
Drug Standards
Standard-value, reference solutions designed to facilitate accurate chromatographic and mass spectrometry-based toxicology and forensic detection of drugs and pharmaceuticals.
Grade
- (1)
- (3)
- (13)
- (563)
- (3)
- (1)
- (24)
- (35)
Concentration
- (1)
- (1)
- (1)
- (4)
- (9)
- (1)
- (1)
- (12)
- (2)
- (14)
- (1)
- (1)
- (3)
- (1)
- (1)
- (18)
- (3)
- (18)
- (1)
- (2)
- (1)
- (29)
- (2)
- (6)
- (1)
- (1)
- (1)
- (1)
Molecular Weight (g/mol)
- (1)
- (1)
- (1)
- (2)
- (2)
- (1)
- (2)
- (1)
- (2)
- (1)
- (2)
- (1)
- (1)
- (2)
- (1)
- (3)
- (1)
- (2)
- (1)
- (1)
- (2)
- (1)
- (1)
- (2)
- (1)
- (2)
- (1)
- (2)
- (2)
- (1)
- (2)
- (2)
- (2)
- (1)
- (2)
- (2)
- (1)
- (2)
- (2)
- (2)
- (2)
- (1)
- (1)
- (2)
- (2)
- (1)
- (1)
- (1)
- (1)
- (1)
- (2)
- (2)
- (2)
- (1)
- (1)
- (1)
- (2)
- (1)
- (2)
- (1)
- (2)
Percent Purity
- (1)
- (1)
- (1)
- (1)
- (2)
- (20)
- (4)
- (1)
- (1)
- (1)
Industry Type
- (2)
- (10)
- (7)
- (1)
- (7)
Filtered Search Results
Products from some of our suppliers do not display in filtered search results. Please
clear all filters
to see these products.
1
–
15
of
781
results
Ibuprofen Impurity I, Pharmaceutical Secondary Standard, Certified Reference Material, MilliporeSigma™ Supelco™
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards
Acetaminophen Impurity G, Pharmaceutical Secondary Standard, Certified Reference Material, MilliporeSigma™ Supelco™
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards