Learn More
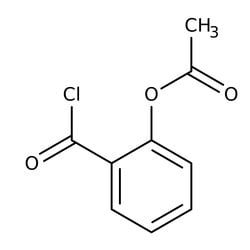
O-Acetylsalicyloyl chloride, 97%
CAS: 5538-51-2 | C9H7ClO3 | 198.602 g/mol
$278.92 - $609.03
Chemical Identifiers
| CAS | 5538-51-2 |
|---|---|
| Molecular Formula | C9H7ClO3 |
| Molecular Weight (g/mol) | 198.602 |
| MDL Number | MFCD00000663 |
| InChI Key | DSGKWFGEUBCEIE-UHFFFAOYSA-N |
| Synonym | 2-acetoxybenzoyl chloride, o-acetylsalicyloyl chloride, acetylsalicyloyl chloride, 2-chlorocarbonyl phenyl acetate, benzoyl chloride, 2-acetyloxy, aspirin chloride, o-acetylsalicyloylchloride, acetylsalicoyl chloride, 2-carbonochloridoyl phenyl acetate, ascc |
| PubChem CID | 79668 |
| IUPAC Name | (2-carbonochloridoylphenyl) acetate |
| SMILES | CC(=O)OC1=CC=CC=C1C(=O)Cl |
| Catalog Number | Mfr. No. | Quantity | Price | Quantity | |||||
|---|---|---|---|---|---|---|---|---|---|
| Catalog Number | Mfr. No. | Quantity | Price | Quantity | |||||
|
AAL0091914
|
Thermo Scientific Chemicals
L0091914 |
25 g |
Each for $278.92
|
|
|||||
|
AAL0091922
|
Thermo Scientific Chemicals
L0091922 |
100 g |
Each for $609.03
|
|
|||||
Description
O-Acetylsalicyloyl chloride was used in the synthesis of enantiomerically pure bidentate heteroorganic ligands built on simple achiral skeletons and containing an aziridine moiety. It was used in the general synthesis of acetoxybenzamides. It was used in the chemical synthesis of 2,2,6,6-tetramethyl-1-piperidinyloxy(TEMPO)-aspirin conjugate via condensation reaction with 4-hydroxy-TEMPO. It was used as reagent in acylation of cyclobutenediones.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
ApplicationsO-Acetylsalicyloyl chloride was used in the synthesis of enantiomerically pure bidentate heteroorganic ligands built on simple achiral skeletons and containing an aziridine moiety. It was used in the general synthesis of acetoxybenzamides. It was used in the chemical synthesis of 2,2,6,6-tetramethyl-1-piperidinyloxy(TEMPO)-aspirin conjugate via condensation reaction with 4-hydroxy-TEMPO. It was used as reagent in acylation of cyclobutenediones.
Solubility
Soluble in toluene. Reacts with water.
Notes
Moisture Sensitive. Store under dry inert gas. Store away from strong bases.
Chemical Identifiers
| 5538-51-2 | |
| 198.602 | |
| DSGKWFGEUBCEIE-UHFFFAOYSA-N | |
| 79668 | |
| CC(=O)OC1=CC=CC=C1C(=O)Cl |
| C9H7ClO3 | |
| MFCD00000663 | |
| 2-acetoxybenzoyl chloride, o-acetylsalicyloyl chloride, acetylsalicyloyl chloride, 2-chlorocarbonyl phenyl acetate, benzoyl chloride, 2-acetyloxy, aspirin chloride, o-acetylsalicyloylchloride, acetylsalicoyl chloride, 2-carbonochloridoyl phenyl acetate, ascc | |
| (2-carbonochloridoylphenyl) acetate |
Specifications
| 5538-51-2 | |
| 134°C to 136°C (12 mmHg) | |
| Pungent, Makes Eyes Water | |
| MFCD00000663 | |
| UN3261 | |
| Moisture sensitive | |
| Soluble in toluene. Reacts with water. | |
| CC(=O)OC1=CC=CC=C1C(=O)Cl | |
| 198.602 | |
| 198.61 | |
| O-Acetylsalicyloyl chloride |
| 47°C to 50°C | |
| >110°C (230°F) | |
| C9H7ClO3 | |
| 25 g | |
| 880372 | |
| 2-acetoxybenzoyl chloride, o-acetylsalicyloyl chloride, acetylsalicyloyl chloride, 2-chlorocarbonyl phenyl acetate, benzoyl chloride, 2-acetyloxy, aspirin chloride, o-acetylsalicyloylchloride, acetylsalicoyl chloride, 2-carbonochloridoyl phenyl acetate, ascc | |
| DSGKWFGEUBCEIE-UHFFFAOYSA-N | |
| (2-carbonochloridoylphenyl) acetate | |
| 79668 | |
| 97% |
Safety and Handling
GHS H Statement
H314-H318-H302
Causes severe skin burns and eye damage.
Causes serious eye damage.
Harmful if swallowed.
P260-P264b-P270-P280-P303+P361+P353-P304+P340-P305+P351+P338-P310-P330-P331-P363-P501c
H302-H314
DOTInformation : Transport Hazard Class: 8; Packing Group: II; Proper Shipping Name: CORROSIVE SOLID, ACIDIC, ORGANIC, N.O.S.
EINECSNumber : 226-899-1
TSCA : No
Recommended Storage : Ambient temperatures
RUO – Research Use Only